Incidence of invasive pneumococcal disease in Finland
- Statistics charts
- Incidence Appendix tables (pdf 301 kb)
- Incidence Appendix tables (xlxs 20 kb)
- Serotype Appendix tables (xlxs 74 kb)
Pneumococci, or Streptococcus pneumoniae, are bacteria that cause upper respiratory tract infections, including sinus and middle ear infections. They may also cause serious diseases that require hospitalisation, including meningitis, pneumonia, bacteraemia and septicaemia.
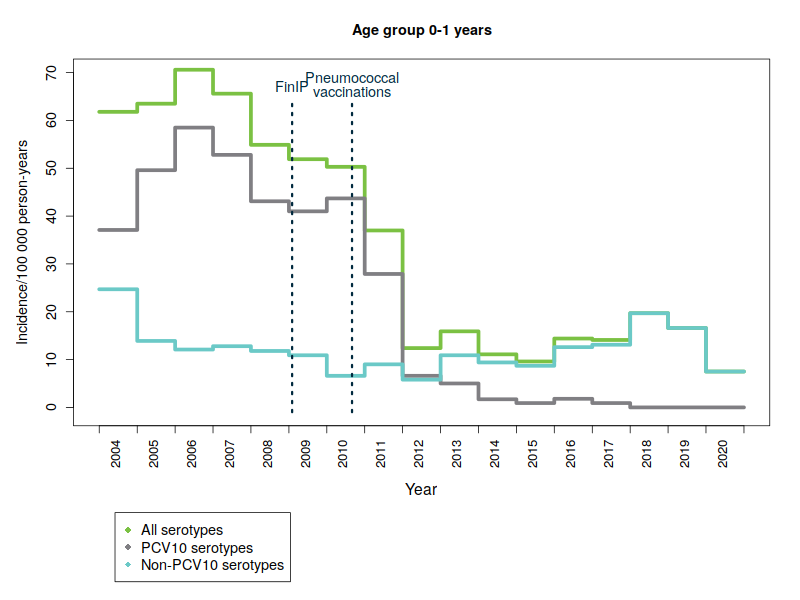
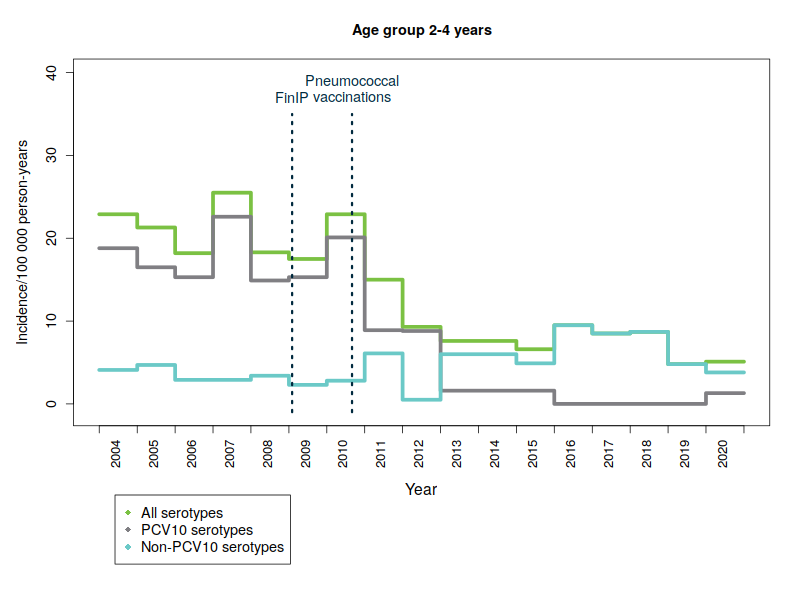
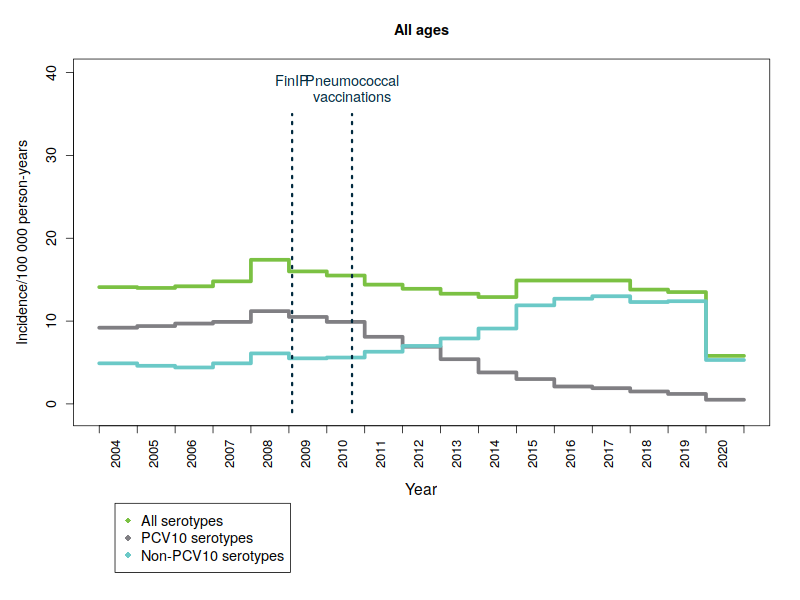
The Finnish Institute for Health and Welfare (THL) is responsible for the surveillance of the incidence of invasive pneumococcal disease, including serotype distribution. The surveillance data are obtained from notifications of infectious diseases that clinical microbiology laboratories submit to the Finnish National Infectious Diseases Register, as well as from bacteriological studies conducted by THL’s specialist laboratory. In Finland, the incidence of laboratory confirmed invasive pneumococcal disease (IPD) has decreased significantly in vaccinated infants and children since the introduction of 10-valent pneumococcal conjugate vaccine (PCV10) into the national vaccination programme in September 2010. All infants and children born in and after June 2010 are eligible for pneumococcal vaccinations through the national vaccination programme. By the end of 2019, children under the age of 5 were diagnosed with 75% less severe pneumococcal diseases on average than children in the same age group before the start of the vaccination programme in 2004-2009.
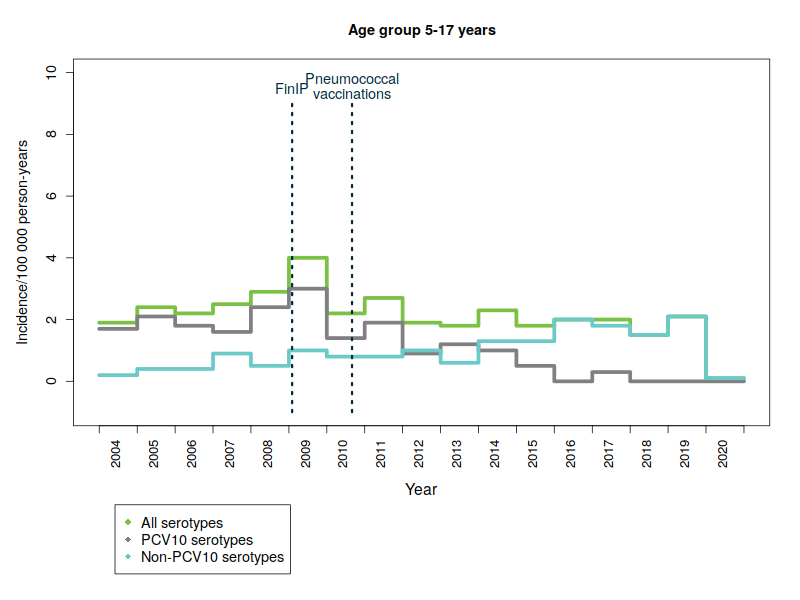
Serious diseases caused by vaccine serotypes have also decreased in the unvaccinated population
One hundred different forms of pneumococcal bacteria – or serotypes – are currently known. The pneumococcal vaccine included in the vaccination programme provides protection against ten common serotypes. Severe diseases caused by pneumococcal types covered by the vaccine have almost disappeared – not only in vaccinated children but also in the unvaccinated population in all age groups. This is due to so-called ‘herd protection’, whereby vaccinated children carry less vaccine-type pneumococci in their throat, which means they no longer pass on the disease caused by these serotypes to other people. Consequently, as a result of a reduction in bacterial infections in vaccinated children, the incidence of disease has also reduced in the non-vaccinated population.
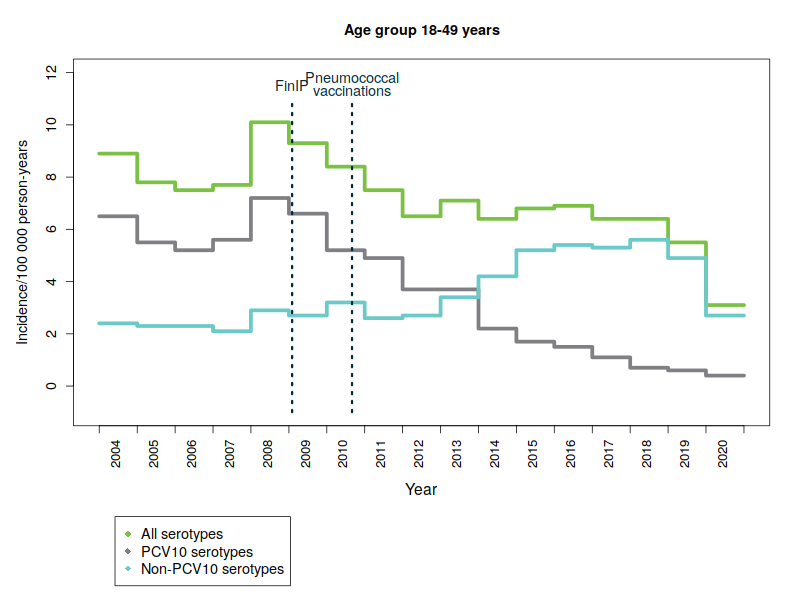
Increased incidence of non-vaccine serotypes in adults
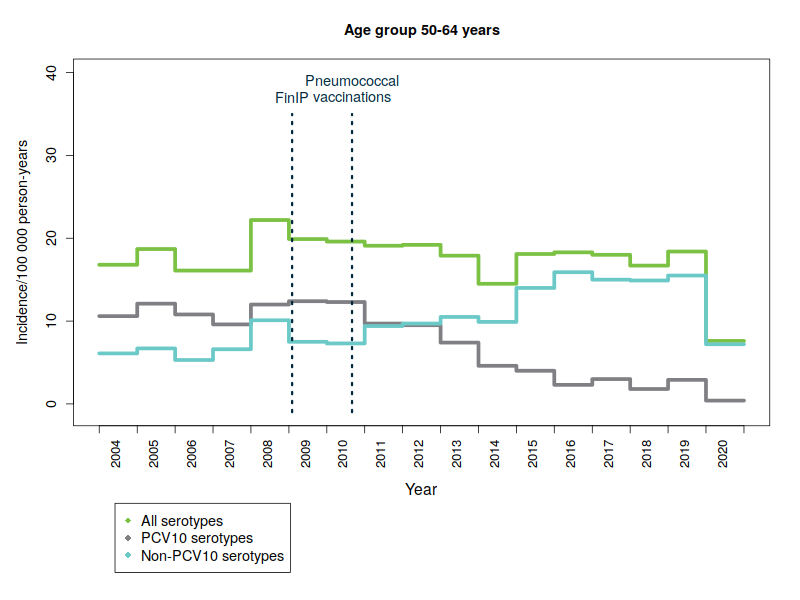
The replacement of serotypes included in the vaccine by other pneumococcal serotypes affects the scope of protection provided by the vaccination. An increase in the so-called replacement diseases has been observed in several countries as a result of vaccinating infants and children. In Finland, the replacement of vaccine serotypes by other types has been low in children. The replacement has been more pronounced among adults, especially those aged over 64. The ageing of population and the increasing prevalence of risk factors for pneumococcal disease may also increase the incidence of pneumococcal disease. A severe flu season can increase the incidence of pneumococcal disease in some years, as influenza is known to predispose to pneumococcal disease.
COVID-19 pandemic containment measures reduced the incidence of pneumococcal disease
In 2020, the incidence of severe pneumococcal disease fell significantly in almost every age group compared to the previous year. It is likely that this was due to the hygiene and containment measures employed and the protective equipment used to control the COVID-19 pandemic, which have also reduced the incidence of other respiratory infectious diseases such as influenza A during the pandemic. The incidence of both serotypes included in the PCV10 vaccine and non-vaccine serotypes decreased.
The figures below show the incidence of pneumococcal disease from 2004 to 2020 by age and serotype group. Please note that the y-axis scale is different for the different age groups because of the variations in disease incidence.

Abbreviations and terms
- PCV10 serotypes: Pneumococcal serotypes included in the national vaccination programme’s 10-valent pneumococcal conjugate vaccine: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F.
- FinIP: a national, community-randomised pneumococcal vaccine study in which about 30,000 children were administered the PCV10 vaccine before pneumococcal vaccinations in the national vaccination programme were begun. Two thirds of the study regions used the PCV10 vaccine.
More information: FinIP. - In graphs, cases for which serotype data are not available have been proportionally divided into pneumococcal serotype-groups.



